
[ad_1]
Europe is rising as a frontrunner within the realm of high quality management (QC) for cell and gene remedy manufacturing, paving the best way for vital developments within the subject. Europe’s dedication to making sure the protection, efficacy, and high quality of this revolutionary remedy is taking part in a pivotal position.
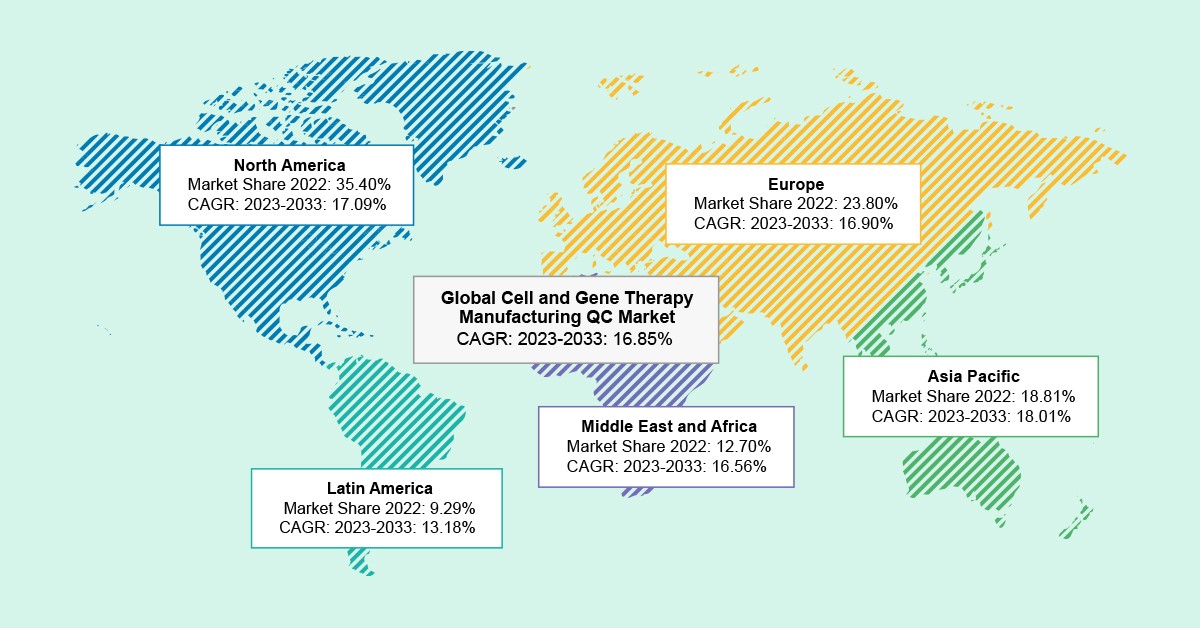
As per the BIS Analysis report, the international cell and gene remedy manufacturing QC market was valued at $1.95 billion in 2022 and is anticipated to achieve $10.65 billion by 2033, witnessing a CAGR of 16.85% in the course of the forecast interval 2023-2033.
Discover extra particulars on this report on this FREE pattern.
Europe will not be solely bolstering affected person confidence but additionally fostering a good atmosphere for the analysis, growth, and commercialization of this transformative remedy.
Dynamic Impression of High quality Management in Cell and Gene Remedy Manufacturing in Europe
The U.Ok. held the biggest market share within the cell and gene remedy manufacturing QC market in Europe, representing 35.07% in 2022. Moreover, it’s anticipated that the QC in France will expertise a progress price of 18.67% from 2023 to 2033, indicating vital growth throughout the forecast interval (2023- 2033).
Europe’s dedication to advancing the sector of therapeutic analysis and growth is clear by its elevated funding within the sector. This dedication is essentially pushed by the presence of distinguished corporations, together with Sartorius AG, Merck KGaA, bbi-biotech GmbH, and Endress+Hauser Group Companies AG (Analytik Jena GmbH).
These are famend gamers within the international business for offering high quality management options in cell and gene remedy manufacturing. By harnessing the experience of those established corporations, Europe is poised to solidify its place as a frontrunner in pharmaceutical manufacturing.
Current Developments in Europe’s CDMO Sector for High quality Management
SCTbio, a contract growth and manufacturing group (CDMO), and Cyto-care.eu GmbH, a specialised firm in superior options for cell remedy and regenerative drugs, entered right into a collaborative settlement in June 2023 aimed toward enhancing the standard of cryopreservation for cell remedy development in Europe.
By way of this collaboration, the businesses search to sort out the demand for streamlined processes encompassing the gathering, cryopreservation, high quality management, and logistics of leukapheresis supplies, thereby supporting totally different facets of the cell remedy workflow.
Moreover, Recipharm, a distinguished CDMO, executed a collection of focused acquisitions to boost its biologics portfolio by welcoming esteemed business specialists Arranta Bio, GenIbet, and Vibalogics into its ecosystem. That is notable for the event and manufacturing of revolutionary merchandise similar to oncolytic viruses, gene therapies, and vaccines, contributing to the progress of human well being.
Conclusion
Thus, from strong regulatory frameworks to cutting-edge analytical applied sciences, Europe’s proactive method positions it as a key participant in shaping the way forward for the cell and gene remedy panorama.
to know extra concerning the growing applied sciences in your business vertical? Get the most recent market research and insights from BIS Analysis. Join with us at [email protected] to be taught and perceive extra.
[ad_2]
Source link